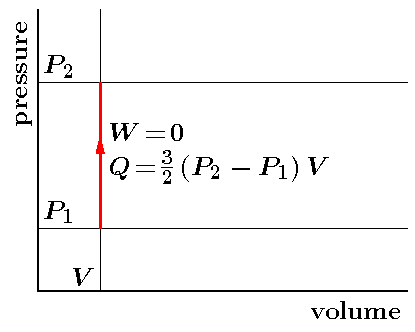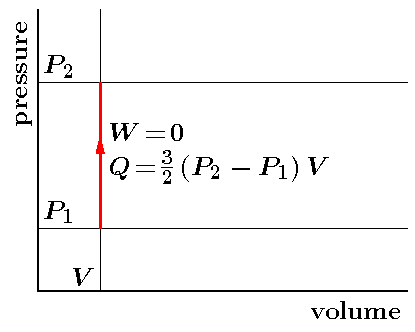previous
next
Fluids
Isochoric process
Let us next return to the isochoric process
which is represented by the figure below.
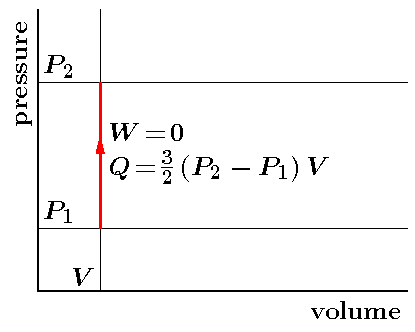
We already saw that while the ideal gas
under a piston which is fixed and cannot move
was heated, it did not produce work.
The total heat required for such process
is only needed for the increase of the total kinetic energy.
Consequently, we have
Q1→2
= Ekin(1→2)
= 3ΔP V/2
= 3(P2-P1)V/2
CV